- Funding Programme
- Year
- 2020
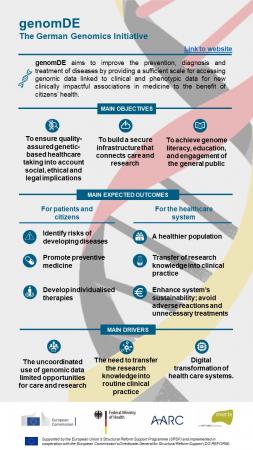
Integrating genomics into healthcare (genomDE)
The European Commission is supporting Germany’s efforts to design a framework for the wider rollout of genomics in healthcare service delivery, specifically in cancer and rare diseases.
Context
Since the Human Genome Project was launched in 1990, it was said that genetics will revolutionize healthcare. It took 13 years, hundreds of scientists and more than 2 billion EUR globally to unveil the complete sequence of our human DNA back in 2000. Now, it takes one or two days to sequence an entire genome for an affordable price. We are now at a point where genomic sequencing is ready to be implemented in routine healthcare. 22 Member States including Germany have signed a declaration on '1+ Million Genomes' Initiative’ to sequence at least one million genomes in the EU by 2022. '1+ Million Genomes' Initiative is catalysing the uptake of genomics and integration of personalised medicine in the European healthcare systems. More than 40 countries joined forces in the International Consortium for Personalised Medicine (ICPerMed), with a common goal of aligning and supporting efforts on personalised medicine research and implementation into healthcare. ICPerMed is supported by the European Commission and managed by Germany.
The German Federal Minister of Health set an ambitious plan to establish a national genome initiative (genomDE) to coordinate numerous existing excellent initiatives to improve the opportunities for care and research in the area of cancer and rare diseases. With genomDE, Germany has the objective to position itself as a competitive player at the highest international level and to implement and establish medical genome sequencing into healthcare. In addition, genomDE will enable optimal use of genomic data for research, thereby realizing the full potential of genome sequencing for scientific progress in biomedicine.
Support to be delivered
genomDE will entail a legal and ethical framework, including a concept for organisation, data infrastructure and reimbursement, as well as a communications campaign aimed at both the public and healthcare professionals.
Expected results
- The capacity of national stakeholders to implement a functional and sustainable genomDE is improved
- The legal and regulatory framework of the genomDE initiative is defined and agreed
- (Stakeholder’s) awareness about the benefits of the genomDE initiative is improved
Project in the spotlight
This project is an example that can be used to encourage other EU Member States to take inspiration from.
Have a look to the factsheet to find out more about the project!